Recent Advances in Neuroimaging of
Multiple Sclerosis and Related Demyelinating Disorders
Neuroimaging Clin N Am 2024 Vol. 34 Issue 3 Pages xv-xvi
Cognitive Impairment in Multiple Sclerosis
Past, Present, and Future
Sarah. A. Morrow
Neuroimaging Clin N Am 2024 Vol. 34 Issue 3 Pages 469-479
Advanced Brain Imaging in Central Nervous System Demyelinating Diseases
A. Cagol, C. Tsagkas and C. Granziera
Neuroimaging Clin N Am 2024 Vol. 34 Issue 3 Pages 335-357
New Imaging Markers in Multiple Sclerosis and Related Disorders: Smoldering Inflammation and the Central Vein Sign
C. C. Hemond, M. I. Gaitan, M. Absinta and D. S. Reich
Neuroimaging Clin N Am 2024 Vol. 34 Issue 3 Pages 359-373

Introduction:
The advent of magnetic resonance imaging (MRI) in the 1980’s revolutionized our ability to diagnose MS and to evaluate responses to treatments. MRI techniques have evolved greatly since that time, providing even greater ability to detect structural changes in the central nervous system as well as brain function and even metabolism.
This blog will discuss some of the most recent advances in MRI technology and how they provide important new insights into disease processes and the evaluation of disease-modifying therapies. In particular I will review how recent advances in MRIs provide insights into central nervous system changes associated with altered cognition, provide insights into newly noted pathologies contributing to progressive multiple sclerosis, and aid in assuring that the diagnosis of MS is accurate.
Key Points:
1. Cognitive impairment begins early in the course of MS and can be a major cause of disability. It correlates weakly with physical disability or numbers of MS lesions in central nervous system white matter (the regions containing nerve fibers or axons). However, as MRIs became more powerful, i.e. with stronger magnets, it became clear that MS was not only an illness affecting white matter, but also resulted in significant changes in both gray matter (i.e. areas with high concentrations of nerve cells), and in tissue layers surrounding the brain, called the meninges.
2. One of the best described MRI-based correlates of cognitive impairment is loss of thalamic tissue, i.e. thalamic atrophy. The thalamus is a major center where nerve fibers from many regions of the central nervous system converge and connect to other areas. Loss of tissue in the corpus callosum, the major connection between the two halves of the brain is also associated with a higher risk have cognitive impairment.
3. The ability to see central nervous system details increases with increasing strength of an MRI’s magnet. The usual magnet strengths of MRIs in patient-care facilities, measured in units called Teslas, is 1.5 Tesla and 3.0 Tesla. It was only when 7 Tesla MRIs became available that researchers were able to assess the full extent of gray matter involvement and meningeal inflammation, present from onset of MS, and becoming more prominent as persons entered the progressive of their illness. Subsequent studies then showed a strong correlation between numbers of cortical lesions, cortical atrophy and cognitive impairment . Impairment was even greater in persons with associated inflammation of the meninges.
4. In addition to the availability, albeit still limited, of stronger MRIs, multiple new imaging software algorithms such as double inversion recovery (DIR) and phase-sensitive inversion recovery (PSIR), increased the ability to see previously unseen areas of tissue destruction.
5. There are several implications of the above findings. First, is that there is no single area responsible for cognitive change. Second, as a result of dysfunction in the areas associated there is an increased risk of developing cognitive impairment, resulting in a loss of connections between neural networks. This results in impaired processing speed, impaired executive abilities, decreased verbal fluency, and shortened working memory. Third, since cognitive impairment can occur early in MS, stronger MRIs and utilization of new software algorithms can identify individuals at greatest risk for developing cognitive impairment and if present, therapies can be instituted addressing this issue early in the course of disease .
6. As noted above, new imaging techniques have greatly enhanced the ability to detect structural changes in the central nervous system of persons with MS . It’s beyond the scope of this blog to detail the specifics of these imaging algorithms, but for those so inclined, the paper by Cagol et al (see above) describes them in detail.
7. Some of the specific structural and biochemical changes now identifiable with the new techniques include a greatly improved ability to detect: a) atrophy or loss of brain tissue, b) regions of myelin loss and myelin reconstitution, c) integrity of nerve fiber (axonal) pathways d) areas of functional neuronal network connections and network connection loss, e) the chemical composition of areas of brain injury and healing, f) subtle regions of gray matter involvement, g) subtle and diffuse structural changes in normal appearing white matter, and g) using radioactive markers to identify cell populations in regions of tissue destruction and remodeling.
8. While about 85% of persons with MS present with relapsing forms of multiple sclerosis, and current disease-modifying therapies significantly reduce numbers of relapses, progression of disability can continue, a phenomenon called PIRA (progression in the absence of relapse activity - see my blog “Changing Insights Demand Change”). Stronger MRIs and new MRI techniques have provided important insights into previously unappreciated pathologic changes believed to be responsible for relapse-unrelated progression, to date all resistant to current immune modulating therapies.
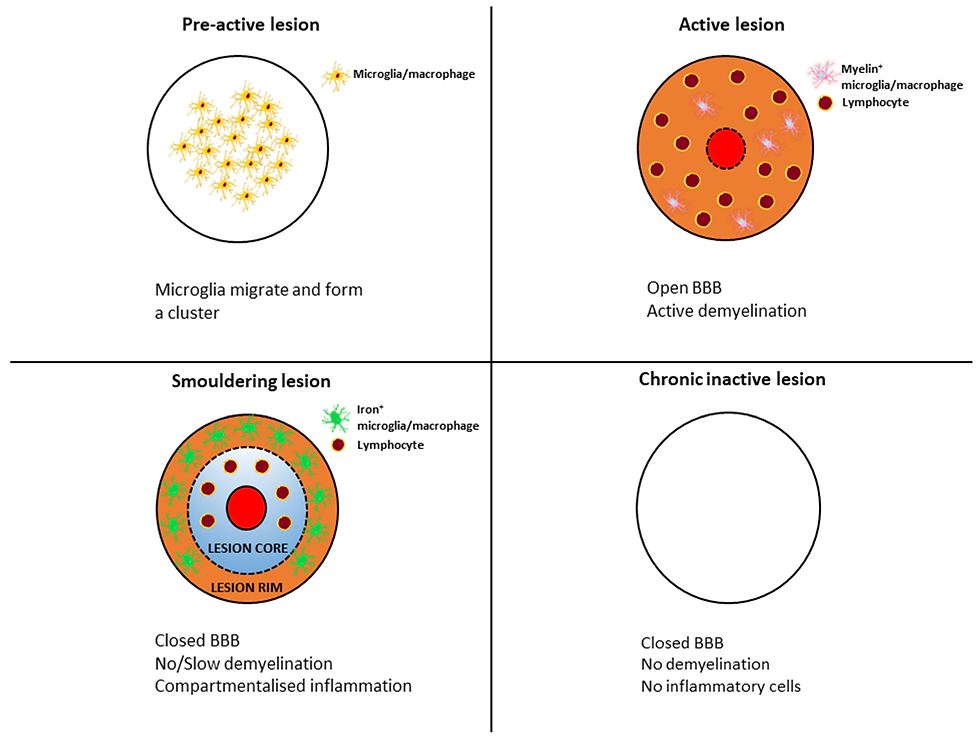
9. The particular changes strongly correlating with disease progression and now visualized with new techniques and MRIs are a) increasing numbers of subtle cortical gray matter lesions b) slowly expanding destructive lesions in white matter and gray matter with rims of iron released from destroyed oligodendrocytes, lesions that are called “paramagnetic rim lesions”, c) slowly expanding lesions without paramagnetic rims, d) inflammation of the tissues covering the brain (“leptomeningeal enhancement”), and e) slowly progressive whole brain and regional brain tissue loss (atrophy), All these phenomena indicate that progression of disease in the absence of relapses is the result of this chronic “smoldering inflammation.”
10. Despite well-characterized guidelines, about 20% of persons referred to MS specialty centers do not have MS. While clinical and spinal fluid parameters remain critical for the diagnosis, changes on MRIs are also of great value. Notable recent MRI changes that, when present, add to greater preclinical precision in making a diagnosis of MS are the presence of paramagnetic rim lesions and the presence of a vein in the center of white matter and gray matter lesions, called the “central vein sign”.
Discussion:
The evolution of MRIs to machines with stronger magnets, in combination with the development of new imaging algorithms, has changed our understanding of many features of MS. It is now clear that evidence of chronic low-grade inflammation and degeneration is present from onset of the illness, that progression of disability can occur in the absence of relapses, and that MRIs are now able to detect the pathologic substrates of these changes (slowly expanding lesions, paramagnetic rim lesions, leptomeningeal inflammation, regional brain atrophy) with greater ease. The key question, of course, is how will these new findings help in the treatment of MS? There are multiple implications.
First, since development of progressive disease does not occur in all persons with MS, it indirectly provides another bit of evidence that MS may be a syndrome rather than a specific disease, with potentially initial multiple events leading to a final common pathway of tissue destruction.
Second, none of the current disease-modifying therapies for MS affect the progressive phase of the illness, so using current indicators of an effective treatment (reducing relapses and other evidence of acute inflammation) are no longer sufficient. Rather, seeing the effects of potential new therapies on the markers of degeneration noted above, and the effect on progression in the absence of relapses, as well as the development of secondary progressive MS will be needed to prove a therapy’s efficacy.
Third, the presence of markers of chronic, smoldering inflammation, especially early in the illness, can have important prognostic value. Genetic studies of this patient population, presumably different than persons without these findings, could lead to new insights into pathogenesis. There already are data indicating that genes associated with disease severity are different than those associated with susceptibility to MS.
Fourth, identifying individuals with markers of smoldering inflammation early in the course of their illness, perhaps with the addition of artificial intelligence and machine learning, would allow researchers to selectively recruit such individuals in clinical trials geared to evaluate agents meant to address these degenerative processes. This may result in a whole new paradigm of therapeutics, very different from most current efforts which essentially duplicate previous therapies in their attempts to reduce numbers of acute inflammatory events.


Comments